
The Swine Health Information Center, launched in 2015 with Pork Checkoff funding, protects and enhances the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments.

Check out the latest episode of SHIC Talk to hear Executive Director Dr. Megan Niederwerder discuss SHIC’s emerging swine disease focus and plans for 2024 with host Barb Determan.
Research provides critical information and resources to help pork producers as they face emerging disease challenges in their swine herds. Research priorities and topics identified in the SHIC 2024 Plan of Work help the Swine Health Information Center fulfill its mission to generate new intelligence for preventing, preparing, and responding to emerging swine disease threats. SHIC has now issued a formal request for proposals inviting submissions to specifically address 11 of the 36 research priorities and topics published in the 2024 Plan of Work, across three of the Center’s five strategic emphases. Proposals are due March 1, 2024, and will undergo a competitive review process for value to the pork industry by a SHIC working group which will provide funding recommendations.
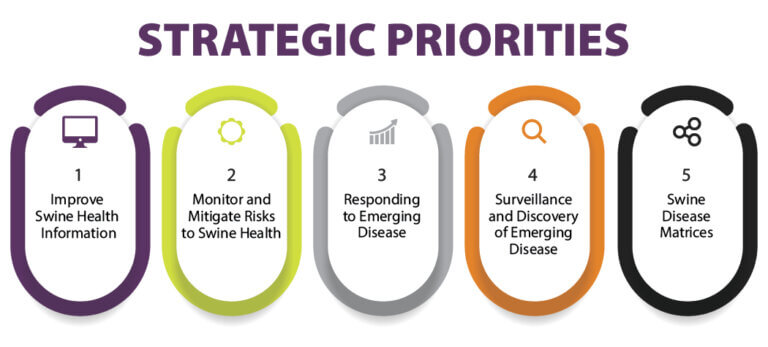
The specific research priorities included in this RFP focus on monitoring and mitigating risks to swine health, responding to emerging disease, and surveillance and discovery of emerging disease. The intent of the RFP is to encourage researchers to develop and submit proposals that specifically address these identified priorities, broaden awareness of funding opportunities to advance SHIC’s 2024 Plan of Work, and to expand the scientific network of researchers and institutions conducting critical research on emerging swine diseases. Funding timely research is an essential component of SHIC providing project outcomes that drive action for emerging disease prevention, preparedness, and mitigation.
Proposals should clearly state the targeted priority that will be addressed through the project. Collaborative projects that include the pork industry, allied industry, academic institutions, and/or public/private partnerships are highly encouraged. Projects that demonstrate the most urgent and timely completion, provide the greatest value to pork producers, and show efficient use of funds will be prioritized for funding. Projects are requested to be completed within a 12-month period with justification being required if the project duration extends beyond 12 months.
Funding available for the SHIC 2024 Plan of Work Research Program priorities included in this RFP is $1.1 million. Individual awards are anticipated to be between $50,000 and $150,000. Budgets exceeding this range require sufficient justification. Projects should strive to be unique, have a high impact, and have industry-wide benefit.
The deadline for proposal submission is 5:00 pm CST on March 1, 2024. The proposal template and instructions for completion and submission can be found at www.swinehealth.org/call-for-research/. For questions, please contact Dr. Megan Niederwerder at [email protected] or (785)452-8270 or Dr. Lisa Becton at [email protected] or (515)724-9491.
RFP Research Priorities as Included in SHIC’s 2024 Plan of Work
Monitor and Mitigate Risks to Swine Health
CBP and high-risk product importation and traveler entry at borders.
Cull sow and secondary market biosecurity and disease surveillance.
Engineering biosecurity controls through site construction design or strategic renovation.
Defining spillover risks of emerging diseases from wean-to-market pigs to sow herds.
Respond to Emerging Disease
Improving diagnostic tools, understanding of pathogenesis, and interpretation of test results for porcine circovirus types 2, 3 and 4.
Identification of early disease outbreak warning signals from industry data.
Diagnostic assay development for confirming efficacy of cleaning and disinfection protocols.
Surveillance and Discovery of Emerging Disease
Population based sample types (oral fluids, processing fluids) for emerging disease testing.
Pan-diagnostic assay development for co-infections or identifying unknown emerging diseases.
Environmental sample types for emerging disease surveillance and efficacy of cleaning and disinfection protocols.
Investigate the clinical relevance and epidemiology of newly identified agents in VDL submissions associated with swine disease.

Porcine circovirus associated disease presents a significant challenge to swine health and pork production globally. Multiple types, including PCV2 and PCV3, have worldwide distribution. An emerging porcine circovirus, PCV4, was reported for the first time in Europe in late 2023 after being initially detected in Asia in 2019. PCV is routinely monitored by the Swine Disease Reporting System for trends in diagnostic submissions, sample types, and test results. Recent trends in PCV2 and PCV3 detection in US herds have raised questions regarding surveillance strategies, clinical presentation, interpretation of results, and control strategies for mitigation of PCV-associated disease. To review trends and emerging issues regarding PCV, the Swine Health Information Center in collaboration with the American Association of Swine Veterinarians will host a webinar on PCV2/3/4 today, February 6, 2024, from 12 to 1:30 pm CST.
During the webinar, presenters will provide PCV-related information including an overview of the pathogen, domestic and global distribution, current and new research updates, diagnostic trends, sample types, interpretation of diagnostic results, and practitioner perspectives for disease management strategies.
PCV topics to be covered on the webinar and confirmed speakers to date include:
General disease overview, domestic and global distribution, update on research outcomes, PCV4 status – Dr. Tanja Opriessnig, Iowa State University
Case definition for PCV2 and PCV3, laboratory submission trends, sampling strategies/types, results interpretation, impact of co-infections with other viruses – Dr. Pablo Pineyro, Iowa State University, and Dr. Darin Madson, Iowa State University
Clinical picture, rational for investigation, sampling strategies, mitigation strategies and outcomes – Dr. Chelsea Stewart, Christensen Farms, and Dr. Mark Ladd, Smithfield

In response to a severe 2021 outbreak of Actinobacillus pleuropneumoniae (APP) serotype 15 in Iowa finishers, SHIC funded research to define the risk and mitigation of this emerging swine disease strain. Drs. Marcelo Almeida and Alyona Michael, Iowa State University, recently completed three objectives as part of the one-year research project, including 1) understanding the APP serological status of sows that supplied the finishers involved in the outbreak, 2) characterizing APP persistence in finishers who had recovered from the disease, and 3) comparing the environmental stability of two APP15 strains with an APP1 strain under laboratory conditions. This study provides information regarding the role of sows for the epidemiology of APP, the recommended sample types for accurate diagnosis, and the bacterial stability across different temperatures and surfaces. Through responding to the APP15 strain emergence, this SHIC-funded study adds key information for improved APP surveillance and implementation of biosecurity practices on the farm.
In late November 2021, several Iowa finisher sites across multiple unrelated systems exhibited high mortality, reaching up to 51% in a matter of days due to an outbreak of virulent APP. Clinical signs associated with the outbreak included rapid onset of high fever, coughing and respiratory distress with mortalities exhibiting frothy, red discharge from both the nose and mouth. This outbreak challenged several assumptions about APP dynamics in the US, such as the prevalence of virulent strains, the risk factors for APP lateral transmission, and environmental persistence. Preliminary sequencing efforts of APP isolates did not identify a clear source herd and further investigation into APP15 was warranted.
Objective 1 of the investigation included cross-sectional sampling to determine the serologic status of sow farms supplying pigs to the APP15 affected finisher sites. Serum samples, nasal swabs, and tonsil scrapings were collected from 30 sows (parity 0 and 1) in each sow herd. Serum samples were tested for APP15 antibodies at the ISU Veterinary Diagnostic Lab, using routine procedures. For the sow farms that submitted samples, 15 of 19 farms were serologically negative for APP serogroup 3-6-8-15 suggesting the majority of sow farms providing pigs for sites experiencing outbreaks were free of APP15. The sites receiving pigs from those sources may have been laterally infected with APP15 during the post-weaning period. Unfortunately, incomplete serologic screening of farms and lack of culture and sequencing follow-up made it impossible to confirm whether the isolate originated from an endemically infected sow farm, as opposed to originating from an alternative source of lateral transmission.
For Objective 2, a prospective longitudinal study of individually identified finishers was conducted at one Iowa site following a confirmed recent APP15 outbreak. Individually identified pigs were repeatedly sampled weekly to evaluate APP15 persistence in the nasal cavity and tonsils and monitor the development of humoral immunity. These results were compared to oral fluids and environmental swab samples to understand population shedding dynamics and environmental persistence. Environmental swab samples included internal locations including feeders, water nipples, and floors and external locations including rendering area and office door handles.
During the finisher investigations, tonsil scrapings had a higher detection rate than nasal swabs or oral fluid, with positive detection in 95% of sampled pigs across six weekly sampling events ending at eight weeks post-outbreak. PCR of tonsil scrapings were overwhelmingly the most sensitive means of screening for individual carriers long-term. APP detection rate in oral fluids was over 10% by PCR up to eight weeks after the reported outbreak. These results are significant in that they suggest that oral fluids have a more temporally robust utility for post-outbreak population surveillance than previously reported.
Environmental sampling for APP15 genetic material was primarily detected in avenues of human traffic (door handles, barn entry floor) and deadstock collection sites (rendering pile). Except for week eight, APP was not detected by PCR in any feeders or waterers. None of the PCR-positive environmental samples yielded isolates on culture. The environmental viability of APP could not be adequately determined due to the high degree of environmental bacterial contamination and further laboratory investigation of APP viability was performed.
Objective 3 compared the longitudinal viability of APP15, for outbreak and historical strains, to APP1 at different temperatures, surfaces, and organic matrices. Survival of each strain on different surfaces (concrete, stainless steel, and rubber) and in different substrates (water, fecal slurry, and horse serum-NAD) was evaluated at six time points post-inoculation (four and eight hours, one, two, three, and seven days) at four temperature set points (-20°C, 4°C, 25°C, 37°C).
For the laboratory comparison of APP serotypes, this study is the first to observe differences in stability across serotypes and the impact of solid substrates on stability when exposed to cooler or freezing conditions. Concrete surfaces exhibited the longest stability for APP15 and APP1 with all strains surviving up to seven days post inoculation at -20ºC, and up to 48 hours at 4ºC with APP1 and APP15h surviving up to 72 hours. Rubber and stainless-steel surfaces exhibited viability at 4ºC and 25°C, but not at 37°C. The results of this benchtop study support previous APP persistence research showing decreased stability in warmer conditions, with relatively prolonged persistence at 4°C and -20°C.
While the outbreak strain of APP15 did not exhibit enhanced persistence compared to other serotypes, contributing factors to the spread and geographic persistence of this bacterium could include PCR detection at rendering piles, increased survival on concrete and rubber surfaces under laboratory conditions, and cold ambient temperatures during the winter 2021 outbreak. Together, the studies reported here generate new important knowledge related to APP ecology and epidemiology that can have important implications for disease diagnosis, monitoring and surveillance, and biosecurity practices.

In December 2023, SHIC funded a study to investigate porcine astrovirus 4 as an emerging pathogen capable of causing respiratory illness in pigs. Dr. Mike Rahe, North Carolina State University, leads the collaborative investigation to define the pathogenesis of PoAstV4 infection in weaned pigs under experimental conditions. Previous studies have shown evidence of PoAstV4 in respiratory tract lesions but the direct contribution of PoAstV4 to clinical disease is poorly understood. The newly funded study seeks to understand if PoAstV4 is a primary cause of respiratory disease, characterize the course of infection over time, and provide a foundation for rapid and accurate identification of respiratory illness in pigs. Investigating the production impacts of potentially emerging pathogens is part of the SHIC mission to define risks to herd health and minimize disease impact.
In a 2022 SHIC-funded study, a retrospective analysis of porcine respiratory disease cases submitted to the Iowa State University VDL from January 1, 2019, to August 1, 2022, was conducted to evaluate the presence of PoAstV4 within microscopic lesions consistent with viral infection of airways. PoAstV4 RNA was detected in affected respiratory epithelium in 73% (85/117) of evaluated cases. This was the first report associating PoAstV4 with respiratory pathology in neonatal and weaned pigs. The next step of research is to define the ability of PoAstV4 to cause clinical respiratory disease and associated lung lesions in naïve pigs.
In the 2023 SHIC-funded study, a collaborative effort between researchers at North Carolina State University, the USDA National Animal Disease Center, Iowa State University, and University of California Santa Cruz, work is underway to evaluate PoAstV4 as a causative agent of respiratory disease in high health, colostrum deprived, caesarean-derived pigs. Utilizing these high health pigs avoids potential exposure to PoAstV4 and the transfer of PoAstV4 maternal antibodies from the sow to piglets. Pigs will be evaluated for the presence of clinical respiratory disease, evidence of gross and microscopic lesions, and presence of virus in multiple tissue types. Characterizing aspects of infection such as location of lesions, duration, and peak virus quantity in tissues over time helps to inform veterinarians how to sample suspect cases for accurate diagnosis. Further, analysis of the immune response to PoAstV4 under a controlled setting will provide information on antibody production.
This information will be important to assist veterinarians in the accurate diagnosis of PoAstV4 in pigs showing clinical signs of respiratory disease without a known etiology. Knowledge gained from the one-year study will be shared with producers and veterinarians as soon as it becomes available.

The Swine Health Information Center leveraged a collaborative partnership with the Foundation for Food & Agriculture Research and Pork Checkoff to fund the 2-year $2.3 million Wean-to-Harvest Biosecurity Research Program currently underway. The first and second rounds of proposal solicitation, selection, and funding are complete. SHIC, FFAR and Pork Checkoff now remind researchers that proposals continue to be accepted for priorities not adequately addressed in funded projects to date. Proposals are invited for submission and funding consideration to investigate cost-effective, innovative technologies, protocols, or ideas to enhance biosecurity during the wean-to-harvest phase of production.
The ongoing research priorities focus on site and transportation biosecurity and cover five targeted areas – 1) personnel biocontainment and bioexclusion, 2) mortality management, 3) truck wash efficiency, 4) alternatives to fixed truck wash, and 5) packing plant biocontainment. SHIC seeks novel tools across all five areas to help result in comprehensive biosecurity enhancement.
Continued researchable interests include:
Proposals must identify and include which of the research priorities is being investigated. They are expected to define current practices and investigate innovative and novel protocols or technologies that may have a cost, efficiency, or implementation advantage. Herd health status monitoring, instead of disease outbreak incidence, can be used to demonstrate success of the protocols or technologies and aid in a required economic analysis of cost-effectiveness. Collaborative projects that include pork industry, allied industry and/or academic public/private partnerships, which demonstrate the most urgency and timeliness of completion and that show efficient use of funds, will be prioritized for funding. If project duration is extended to assess seasonal effects, a justification for the timeline should be clearly stated. Proposals are capped at $200,000.
All proposals should be submitted via email to [email protected] using the proposal template and instructions found here. Questions can be directed to Dr. Megan Niederwerder at [email protected] or Dr. Lisa Becton at [email protected].

This month’s Domestic Swine Disease Monitoring Report brings the new chart for weekly monitoring of Influenza A virus PCR detection. The newly implemented monitoring capability provides the industry with information about IAV detection and enables comparison to the historical expected for a specific period. Also, the report brings information about the decreased PRRSV positivity in all age categories, breaking the four consecutive months of increase in the wean-to-market positivity category. However, the average PCR Ct values of the submissions remain low. For the PRRSV ORF 5 sequence, the L1C lineages (L1C.2, L1C.3, L1C.5, L1C-others) remain predominantly detected in the field, and in 2023 accounted for 53% of all wild-type ORF5 sequence detections. For enteric coronaviruses, PEDV positivity had a sharp increase during January in all age categories, raising an alert for the activity of this pathogen. Also, PDCoV had a moderate increase in wean-to-market positivity. Actinobacillus suis entered the top 10 confirmed tissue diagnoses for the first time in the monthly report at ISU-VDL. In the podcast, the SDRS hosts talk with Dr. Brigitte Mason (Country View Family Farms) about the movement of PRRSV L1C.5 (variant) to the eastern corn belt states and disease management in the winter months.

African swine fever keeps spreading in Europe where Montenegro has become the 27th European country to report the disease for the first time in wild boars. In Italy, the European Commission has officially declared the resolved status of the outbreak genotype II in Sardinia and characterized it as “occasional” and “imported.” Remaining Italian outbreaks are confined to the province of Nuoro. The first report of ASF in domestic pigs in Gyeonggi-do province, South Korea, since April 2021 has occurred. There were 2,448 pigs culled on site in response. Foot-and-mouth disease outbreaks have been reported in six countries in Africa. There is limited information available regarding the responsible viral strains; for only two of those, serotype O has been confirmed. Unprecedented volumes of illegally imported meat have been detected by Pork of Dover, UK, authorities, raising concerns about the risk of ASF. In Naples, Italy, traces of ASFV detected in Chinese salamis falsely labeled as vegetarian were discovered.
Copyright 2024 | Swinehealth.org | Website by Heartland Marketing Group