
The Swine Health Information Center, launched in 2015 with Pork Checkoff funding, protects and enhances the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments.


With the beginning of 2024 comes the anticipated change in leadership for the Swine Health Information Center. Dr. Megan Niederwerder now serves as executive director while Dr. Lisa Becton begins her tenure with SHIC as associate director. Both are eager to carry forward the mission of the Center to protect and enhance the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments.
Dr. Niederwerder joined SHIC in April 2022 as associate director. During her time with SHIC, she has been engaged in all facets of the Center’s operations, projects, and priorities. This experience, in concert with her previous swine industry roles, prepared her for assuming the executive director position. “SHIC strives to provide a unique and valuable service to the US pork industry by focusing efforts on emerging diseases,” Dr. Niederwerder observed. “We will continue to work diligently on initiatives to serve pork producers and their practitioners with emerging disease information efficiently and effectively.”
Under new leadership, SHIC will continue to deliver domestic and global swine disease monitoring information, research to address emerging disease threats, and review of swine health data. SHIC’s nimble structure allows it to address threats to US swine herd health as they arise. The Wean-to-Harvest Biosecurity Program and efforts to prevent and prepare for potential emerging diseases, such as Japanese encephalitis virus, are key SHIC priorities.
“We’re going to have challenges. How quickly we can identify those challenges and turn those concerns into solutions shapes our effort,” remarked Dr. Becton. “A lot of the industry’s focus is on how rapidly we can diagnose new, emerging swine disease threats, and how quickly we can tell if an existing pathogen has changed to create new problems. This is at the core of SHIC’s mission.” In her new role, Dr. Becton brings her previous experience in the industry and devotion to US pork producers to enable solutions.
SHIC’s work in 2024 will be guided by the Plan of Work approved by the board of directors. On-going and new efforts also build on previous work detailed in the 2023 Progress Report. Drs. Niederwerder and Becton welcome swine industry stakeholders to provide ongoing input and ideas to fulfill the SHIC mission and provide return on investment to US pork producers.

New 2023 Progress Report provides pork producers, swine veterinarians, and industry stakeholders a review of the Swine Health Information Center’s activities and accomplishments to carry out its mission over the past year. The Progress Report is divided into sections detailing the year’s progress addressing SHIC’s 2023 Plan of Work priorities – preparedness, monitoring and mitigating risks to swine health, improving swine health information, surveillance and discovery of emerging disease, and responding to emerging disease. Information about the organization, its board of directors, two working groups, outreach to pork producers and all stakeholders, and its communication program is also detailed in the Report.
SHIC activities are done with constant communication and coordination with the National Pork Board, the National Pork Producers Council, and the American Association of Swine Veterinarians to serve the US pork industry. The Progress Report begins with an executive summary that gives a brief description of SHIC and 2023 accomplishments. Also included in the report is a detailed description of the completed USDA-FAS funded ASF research program conducted in Vietnam.
While guided by the 2023 Plan of Work, SHIC also strives for nimbleness to urgently address new industry needs as they are identified. SHIC, along with the Foundation for Food & Agriculture Research, an organization advancing actionable science to develop tools, technologies, and information for farmers, consumers and the environment, and Pork Checkoff, funded the two-year $2.3M Wean-to-Harvest Biosecurity Research Program. The program funded 16 research projects in 2023 and will continue to fund projects into 2024. By leveraging budget allocation with the matching funds from FFAR and the Checkoff, SHIC increased capacity and output for its mission to safeguard the health of the US swine herd.
The SHIC Board of Directors reallocated funds from the 2023 budget projection for collaborative Japanese encephalitis virus research to strengthen US pork industry preparedness for this virus that caused a wide-spread swine disease outbreak in Australia in 2022. The board also designated funds to research the use of tongue tip fluids from pig mortalities to monitor disease circulation in US pig herds, a new need identified in 2023 by a SHIC working group.
SHIC began operation as a 501(c)(3) corporation on July 4, 2015. The mission of SHIC is to protect and enhance the health of the US swine herd by minimizing the impact of emerging disease threats through preparedness, coordinated communications, global disease monitoring, analysis of swine health data, and targeted research investments. When SHIC was formed in 2015 by a grant of Checkoff funds from the National Pork Board, it was with the understanding that it was a five-year project. During 2021, the National Pork Board’s Board of Directors voted to provide $15M to continue to fund SHIC’s work through 2027.
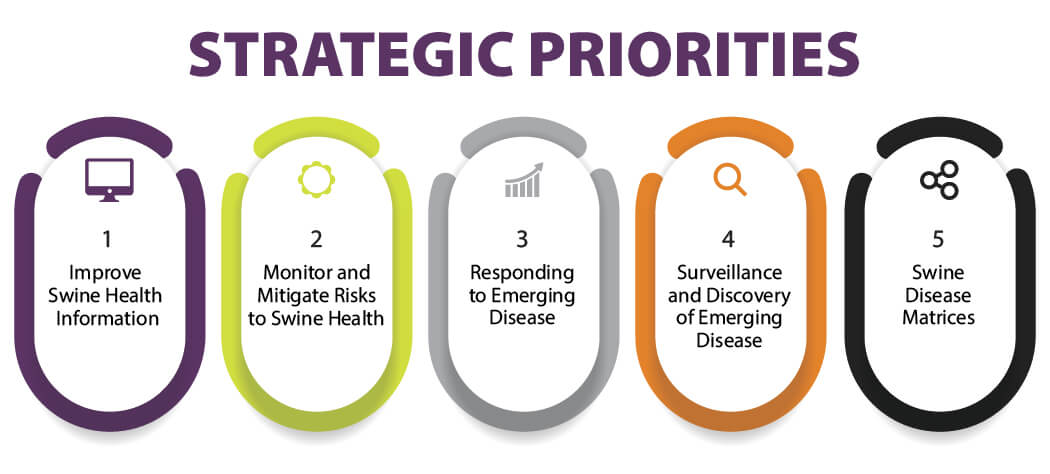
Activities of the Swine Health Information Center are guided by its annual Plan of Work detailing projects to address its five strategic priorities, including 1) improving swine health information, 2) monitoring and mitigating risks to swine health, 3) responding to emerging disease, 4) surveillance and discovery of emerging disease, and 5) swine disease matrices. Developed through stakeholder feedback and approved by the SHIC Board of Directors, the 2024 Plan of Work will be implemented by Executive Director Dr. Megan Niederwerder and Associate Director Dr. Lisa Becton with input from the board and SHIC Working Groups.
Proposals to address the priorities detailed in the 2024 Plan of Work are accepted on a rolling basis for review and funding recommendation. SHIC’s activities are guided by the Plan of Work while remaining nimble and responsive to industry needs. Stakeholder input and ideas are welcomed year-round to inform newly identified needs which may necessitate adapting the Plan of Work to fulfill SHIC’s mission. https://www.swinehealth.org/plan-of-work-input/ Input may include topic areas, research priorities, and identified industry needs in which SHIC should focus efforts, such as an emerging swine disease or an emerging swine health issue.
2024 Plan of Work Highlights
Improve Swine Health Information
Monitor and Mitigate Risks to Swine Health
Respond to Emerging Disease
Surveillance and Discovery of Emerging Disease
Swine Disease Matrices
‡ FFAR/SHIC/NPB Wean-to-Harvest Biosecurity Research Program priorities.

Dr. Chad Paulk, Kansas State University, led a project to understand the best flushing, thermal processing, and decontamination techniques for a feed mill after exposure to PEDV, PRRSV, and SVA. In addition to PCR testing for viral RNA, infectivity of feed and environmental samples collected after utilizing the decontamination techniques were evaluated using swine bioassays. Overall, data showed that chemical flushing, thermal processing, and facility decontamination reduce the quantity of viral RNA, but more research is needed to understand how these techniques affect virus infectivity.
Find the SHIC and feed industry-funded study’s industry summary here and the KSU Feed Mill Biosecurity Fact Sheet including data from project here.
Work to understand feed mill decontamination was motivated by the risk feed and feed ingredients pose as a potential route for introduction and spread of ASFV and other transboundary viruses of concern to the US swine industry. Noting previous research has determined the minimum infectious dose of ASFV in feed (Niederwerder et al., 2019) as well as work showing ASFV can survive in various feed ingredients during transboundary shipping (Dee et al., 2018), Dr. Paulk pursued funding from SHIC, along with the Institute for Feed Education and Research, the United Soybean Board, and the Animal Nutrition Association of Canada for the investigation. The research team recently finished the project and delivered results to help inform the North American swine industry on feed manufacturing facility decontamination processes.
In the project, researchers investigated chemical additives, pelleting of feed, and facility decontamination methods to understand practices which may mitigate the risk of PRRSV, PEDV, and SVA spread from a contaminated feed mill. Researchers reported that formaldehyde flushes (either liquid or dry) were the most effective at reducing viral concentrations in both the feed and the environment; however, Dr. Paulk said eliminating viral RNA from the environment proved to be very difficult.
The complete facility decontamination (removal of organic matter with heated pressure washing, disinfection with 1% Virkon, disinfection with 5% household bleach, environmental heat held at 140°F for 48 hours) was the only decontamination treatment where PEDV, PRRSV, and SVA RNA was non-detectable after completion of all steps. Other treatments, including chlorine dioxide and heat, reduced the quantity of RNA but did not fully eliminate PCR detection of viruses across mill surfaces. Further, thermal processing, pelleting, reduced the detectable RNA in both feed and the environment for all viruses.
Although PEDV and SVA RNA were detectable by PCR in samples post-treatment, these samples failed to cause infection of pigs after inoculation, providing evidence of successful mitigation. However, feed and dust samples collected post-treatment of PRRSV caused infection in pigs post-inoculation, even when the inoculum was negative for PRRSV via PCR. Researchers report it is unknown why PRRSV RNA was not consistently detectable via PCR, but the bioassay suggests more information is needed regarding PRRSV mitigation.
Overall, this study demonstrated the difficulty in eliminating viral RNA from a contaminated feed mill post-introduction and highlights the need for continued emphasis on biosecurity for preventing pathogen entry.

The Swine Health Information Center has awarded research funding to five projects designed to better understand the potential use of tongue tips for monitoring emerging diseases. When announcing the request for proposals on tongue tip research, SHIC’s research priorities included diagnostic test sensitivity and specificity, diagnostic lab processing procedures, virus isolation techniques, use to achieve PRRS stability or elimination as a model for emerging disease, and use for investigating vertical and/or horizontal disease transmission. Overall, more information is needed about how to apply tongue tip monitoring to support producers and veterinarians in recovery from emerging diseases. After a competitive proposal review process, awards were made to the University of Minnesota and Iowa State University in fall 2023.
Titles of the five projects funded are:
These projects will help the industry by providing more information about diagnostic test sensitivity or specificity using tongue tips compared to other sample types such as processing fluids in neonates and oral fluids in nurseries. They will also investigate diagnostic lab processing procedures to support reliable, credible test results, as well as optimizing tongue tip collection protocols for recovery of live virus isolates. With this information, the ability to reliably research pathogens causing emerging disease outbreaks will increase.
Because there is no predicting when or where the next emerging disease will appear, SHIC needs to be prepared with funds in place that can be quickly mobilized to support filling the immediate research gaps following an outbreak. This research will provide producers and their veterinarians with critical information that they will need to effectively respond to the disease outbreak.
With funding from the Swine Health Information Center renewed for 2023-2024, the Morrison Swine Health Monitoring Project’s industry-driven goals continue to focus on enhancing the health of the US swine herd by providing tools that enable implementation of expanded preparedness measures to address emerging or foreign animal disease emergencies. This includes continuously tracking and analyzing trends in the incidence, prevalence, and elimination of pathogens, sustaining ongoing surveillance of PRRSV sequences impacting the US swine population, enhancing producer engagement, broadening representation of the industry, and facilitating access to timely and relevant disease information.
In their proposal for funding, the MSHMP team wrote, “Building on our longstanding dedication to the swine industry foreign animal disease preparedness, we remain committed to the development of tools for the industry through collection, analysis, and reporting of data pertaining to endemic disease incidence and eradication endeavors.” In 2023-2024, the team will explore potential inclusion of new pathogen incidence estimations, specifically Senecavirus A, while ensuring the ongoing accuracy and reliability of MSHMP’s data. MSHMP will continue to actively cultivate relationships and facilitate interactions among participants, as well as foster collaborations between participants and research institutions.
Endemic swine diseases and potential for foreign animal disease introduction pose a risk to the US pork industry. These challenges form the basis for MSHMP’s sustained effort to understand, monitor, and mitigate disease risks. Launched in 2011, MSHMP has made substantial contributions to swine health management in the US by monitoring the health status of individual breeding sites, which collectively represent more than 60% of the US breeding herd. MSHMP has facilitated a more comprehensive understanding of the impact of diseases like PRRS and PED while also uncovering data insights such as spatial and temporal patterns of disease transmission, the molecular evolution of PRRSV, and risk factors associated with disease outbreaks.
MSHMP’s granular data has allowed for the timely detection and detailed description of newly emerging PRRSVs variants such as the Lineage 1C 1-4-4. And new developments within the project will help shape the industry’s response to ongoing disease challenges. For example, MSHMP’s data increases understanding of the endemic transmission of diseases like PED, offering the industry the opportunity to engage in informed discussions about elimination strategies.
Reprinted verbatim from a USDA APHIS email 12/27/2023
APHIS Veterinary Services (VS) is updating the Japanese Encephalitis (JE) Response Strategy and invites feedback from key stakeholders.
JE is not currently found in the United States. The primary purpose of this document is to provide strategic guidance to those government officials responding to an outbreak if it were to be introduced into the United States. This revision updates and expands the scope of the 2013 JE Disease Response Strategy. We have incorporated current science and research, shifted away from eradication as a response strategy, and updated considerations for control and vaccination. We also included more elements of the response than were previously addressed, such as recognizing a one health approach, increasing collaboration with public health authorities, and enhancing communication plans.
Please provide feedback or comments, such as technical information, assessment of operational feasibility, and resource constraints, regarding the response strategy, using this form: https://forms.office.com/g/kwus5M8c5D. The form will be open until Wednesday, January 26, 2024.
If you have any questions, please reach out to the APHIS Veterinary Services Emerging Diseases Coordinator, Dr. Sarah Speth, at [email protected].

This month’s Domestic Swine Disease Monitoring Report brings the 2023 highlights, including the major findings regarding the swine pathogens monitored in the project. Also, the report brings information about the spike in the PRRSV positivity in the wean-to-market category, completing the fourth month of increased positivity. At a regional level, PRRSV overall positivity continues above expected in Iowa, Illinois, and Ohio. For enteric coronaviruses, PEDV positivity increased in all age categories, raising the alarm for upcoming months. At a regional level, PEDV’s overall percentage of positive submissions is above the expected in Missouri and North Carolina. For influenza A virus and PCV2, overall positivity remains similar as in November, with over 33% and 40% of positive submissions, respectively. Even though there were only a small number of cases, there were signals for an increased number of Trueperella pyogenes, Mycoplasma hyorhinis, and PEDV confirmed tissue diagnoses at Iowa State University Veterinary Diagnostic Lab. In the podcast, the SDRS hosts discussed the highlights of 2023, going through the major findings of each pathogen monitored by the project.

In the January Global Disease Monitoring Report, find a 2023 summary including highlights of events across six continents (Asia, Africa, Europe, Oceania, North America, and South America). Events saw over 780,000 pigs directly affected by the outbreaks of seven diseases (ASF, PRV, JEV, FMD, CSF, PED, Nipah). The report also features an in-depth analysis of FMD with an overview of the disease dynamic throughout the year. FMD outbreaks were reported in 30 countries in 2023. Read about Russia resuming pork exports to China after a 15-year break, following the lifting of a ban imposed by Beijing due to an African swine fever outbreak in 2008. Surveillance at points of entry resulted in a new type of ASF being detected at the Taiwanese border for the first time. This new strain – recombinants of genotype I and II ASFVs – was first confirmed in 2022 and has been found in various regions of China, including Jiangsu, Henan, and Inner Mongolia.
Copyright 2025 | Swinehealth.org | Website by Heartland Marketing Group